Oncuria delivers actionable insights for early detection, personalized BCG therapy decisions, and effective monitoring–because precision saves lives.
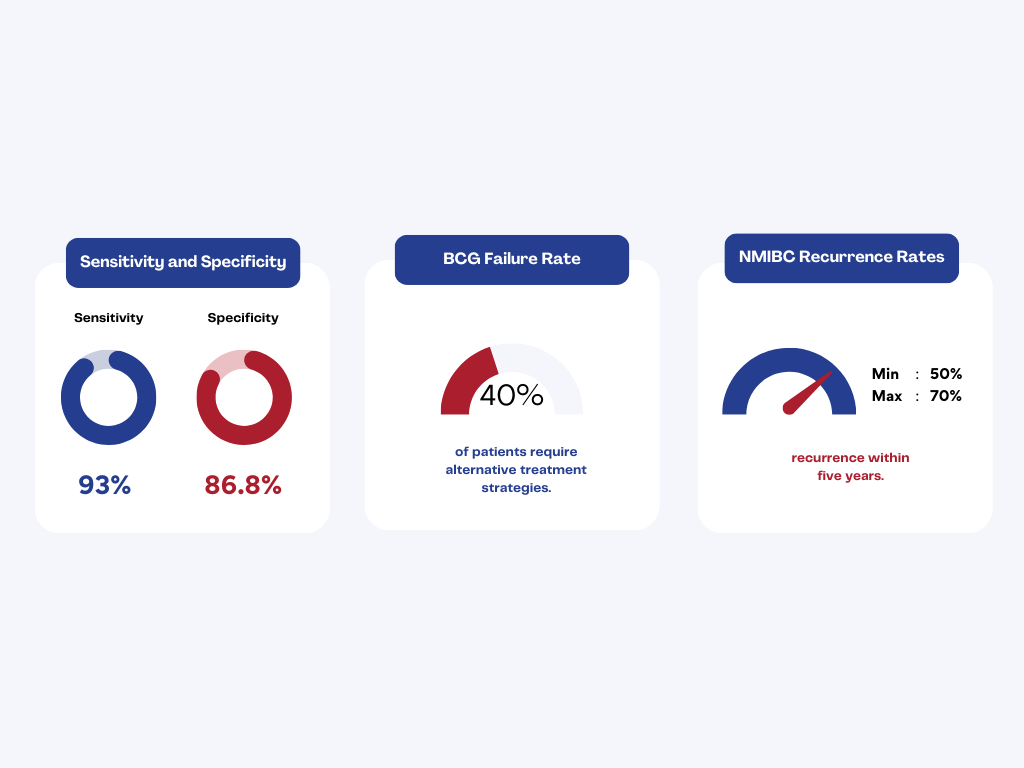
Every year, non-muscle-invasive bladder cancer (NMIBC) affects thousands of patients, yet 30–40% fail to respond to BCG therapy¹.
These delays in identifying recurrence or progression can result in unnecessary treatments and worsened outcomes.
What’s the solution? Precision diagnostics that provide actionable insights when they matter most.
With high recurrence rates and limited BCG therapy availability, a lack of precision in care costs both time and patient well-being. Oncuria changes that.
Oncuria’s urine-based diagnostics empower urologists–like you–with advanced tools to:

Oncuria’s urine-based diagnostics empower urologists–like you–with advanced tools to:
With ongoing BCG global shortages⁴ impacting bladder cancer treatment, Oncuria empowers you to optimize resources while improving patient outcomes confidently:


Use our secure shipping process to send the sample to our lab.

Receive clear, actionable insights to guide your next steps in patient care.
By addressing these challenges, Oncuria helps you deliver better patient outcomes with confidence.
The Oncuria family comprises three proprietary tests:
By analyzing proprietary biomarker panels, Oncuria tests help clinicians:
Yes, Medicare⁸ and most major private and commercial insurance plans cover Oncuria with the appropriate ICD-10 code.
Ordering is straightforward:
No prior authorizations are required—just select the appropriate ICD-10 code.
You’ll typically receive results in the online portal within 5-7 business days.
Results are available within 5–7 business days via the secure online portal. Reports include:
Proper handling ensures sample integrity:
The Oncuria platform evaluates 10 protein biomarkers linked to bladder cancer, focusing on angiogenesis, immune response, and cell proliferation. This biomarker panel enables precise risk assessment and monitoring. [Source: Diacarta Clinical Data]
Yes, Oncuria Detect and Predict tests use biomarker profiling to stratify patients by disease grade and recurrence risk, enhancing the ability to personalize treatment plans.
Speak to one of our representatives today to learn more about our CAP and CLIA-accredited laboratory.
Copyright © 2025 Genetics Institute of America. All Rights Reserved.